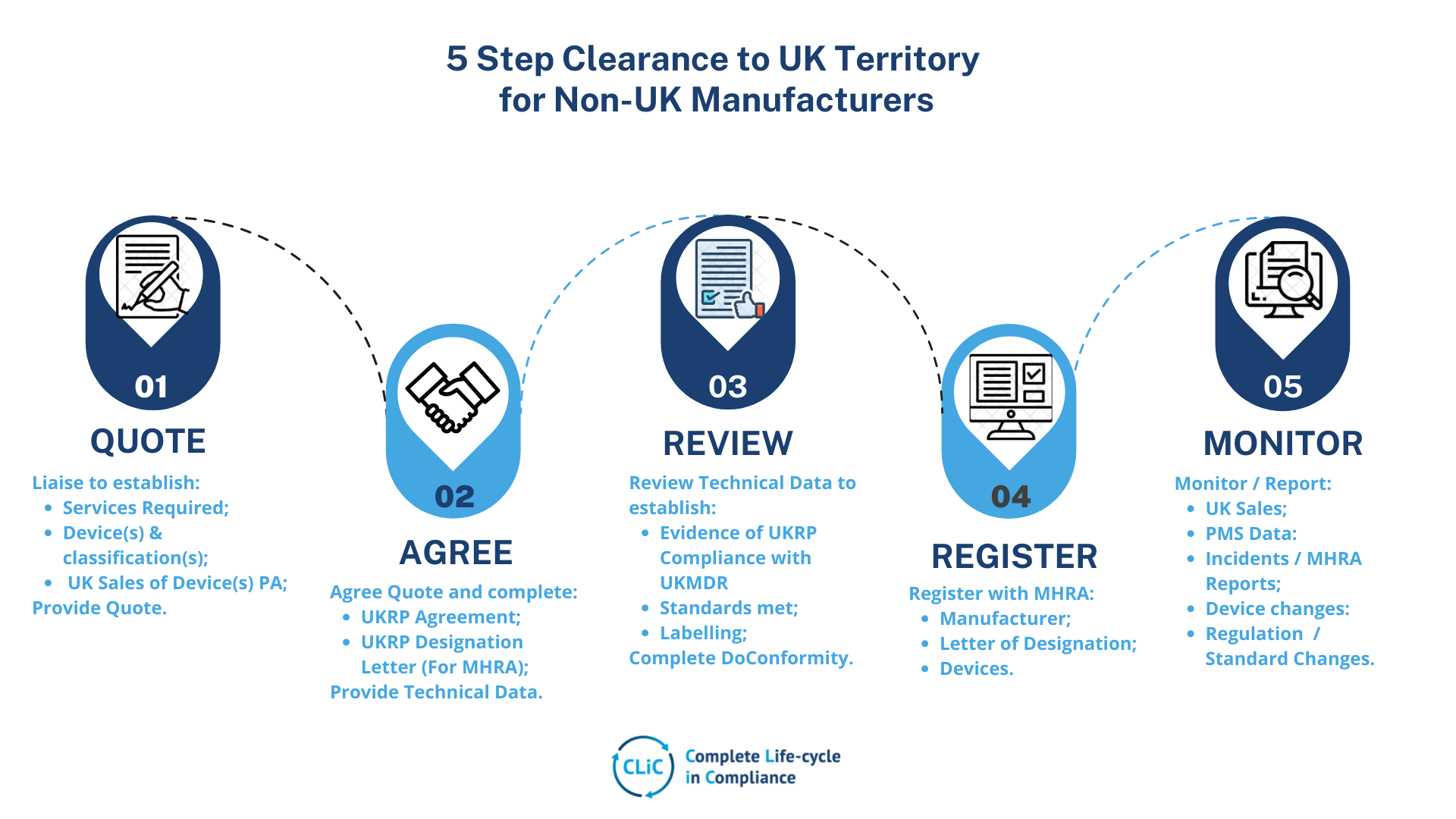
UK Responsible Person (UKRP)
For Non-UK Manufacturers, we provide efficient, experienced, compliant and business focused UKRP solutions and advices to meet:
- UK Medical Device Regulations & standards;
- UKCA and UKNI Marking
- Manufacturer and Devices Registration with MHRA;
- Laison with Importers and Distributors;
- PMS, Vigilance and PMCF.
Register for free discussion
We commit to respond promptly; please complete the enquiry form or contact us on one of the following:

